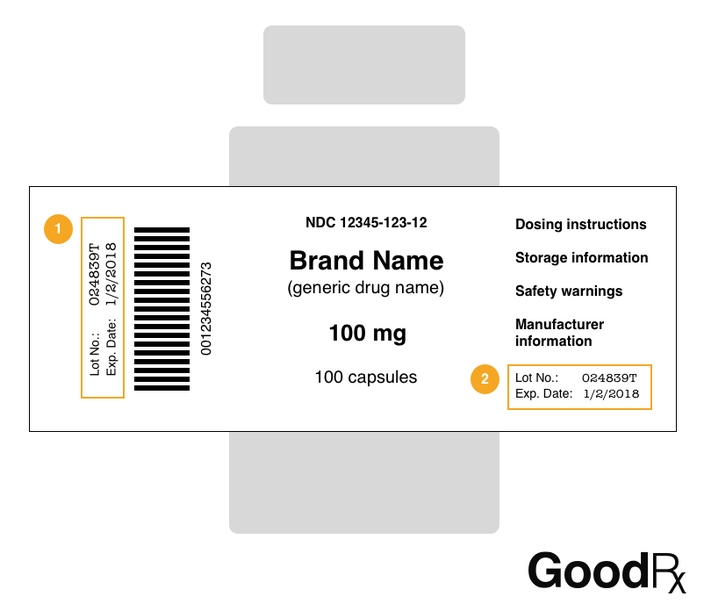
41 medication labels must include
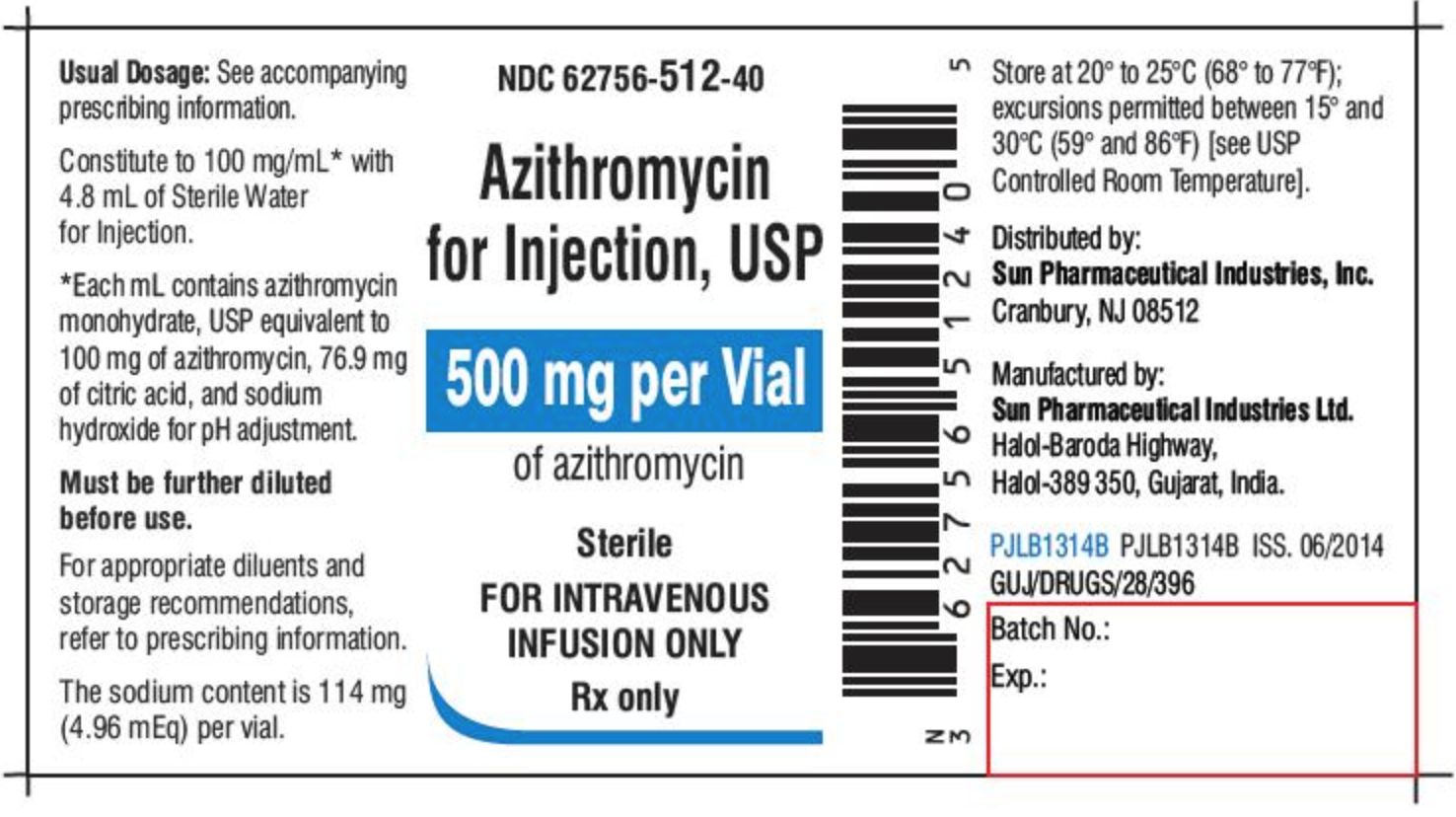
What Information Should Be on Drug Labels? - MedicineNet 3 Feb 2022 — Labeling requirements for prescription drugs · Statement of identity · Brand name · Net quantity of contents · Statement of dosage. Statement on Labeling of Pharmaceuticals for Use in ... Label content: Syringes: The drug's generic name and concentration (in units per mL) should be the most prominent items displayed on the label of each syringe.
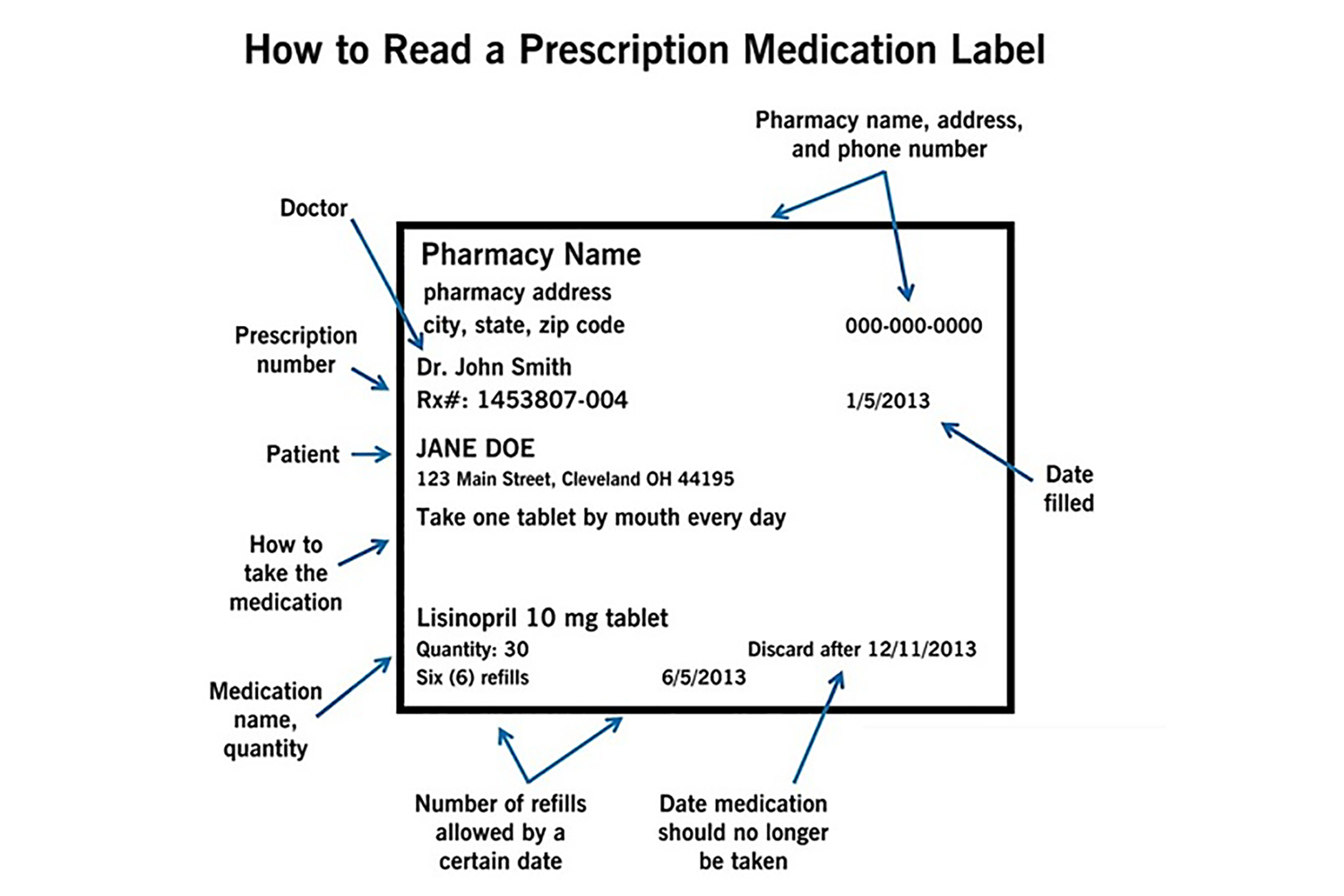
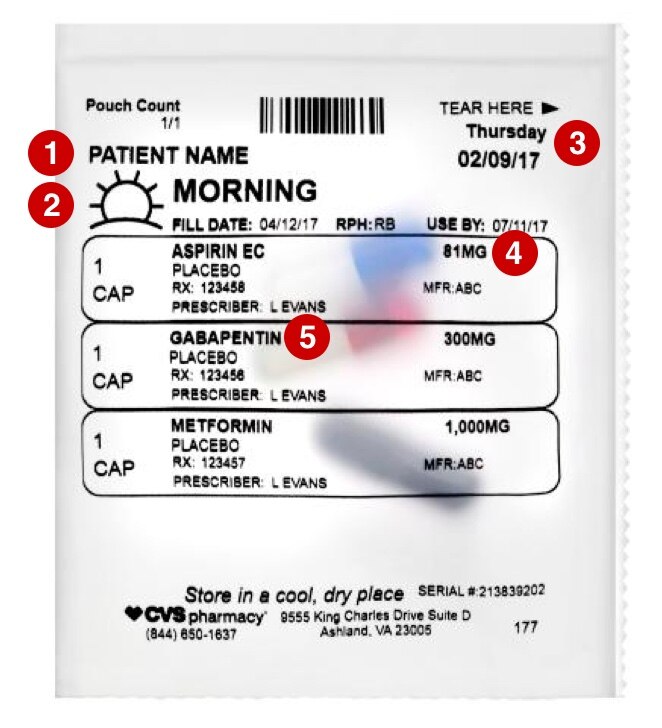
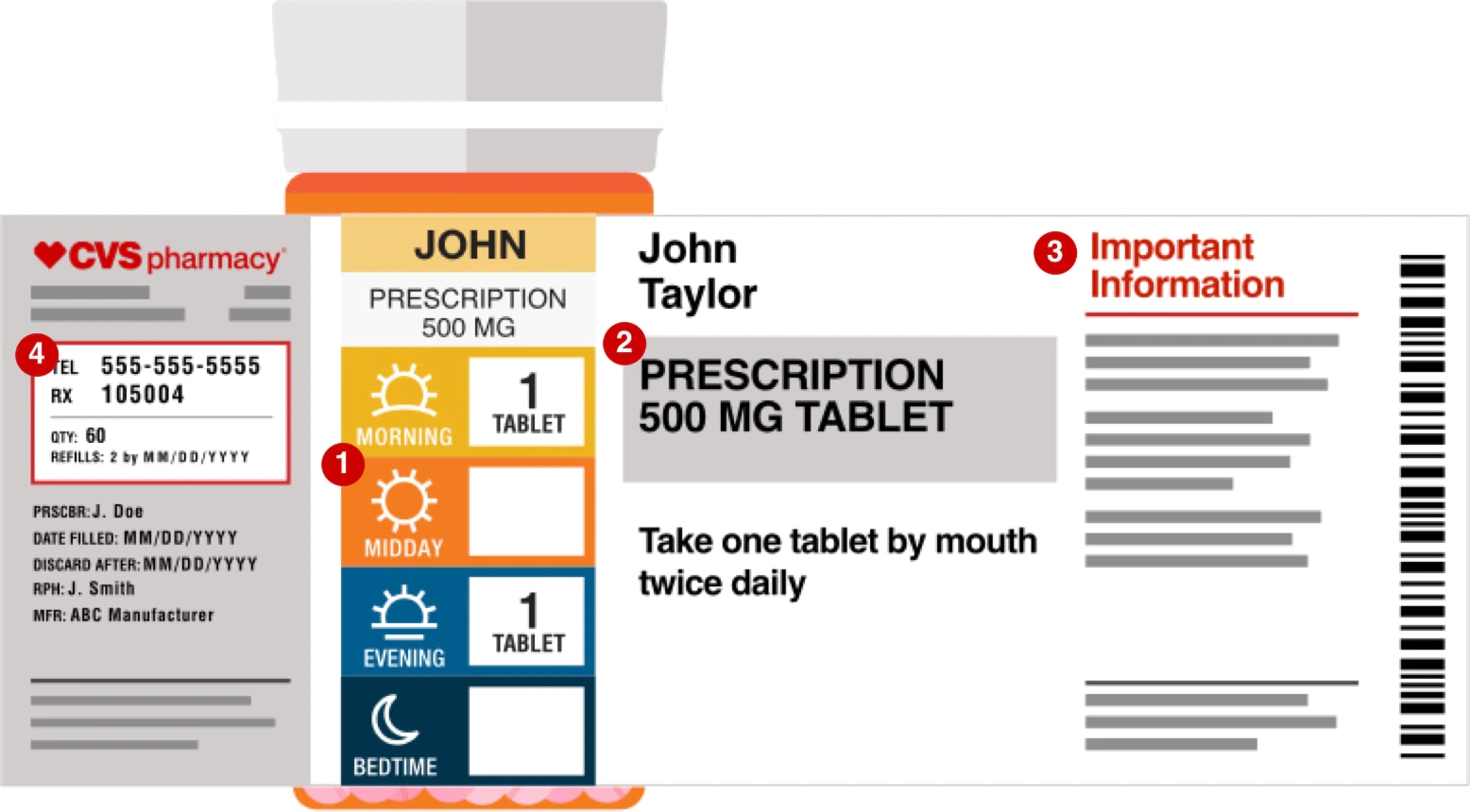
Understanding Prescription Medication Labels - Rx Outreach If it says “three times a day”, take the medication at three intervals, such as morning – afternoon – evening. Always read the warning labels on your prescription (if applies). The warning labels are usually on the side or back and are often separated from the main label. The package insert will describe all side-effects and warnings.

Medication labels must include
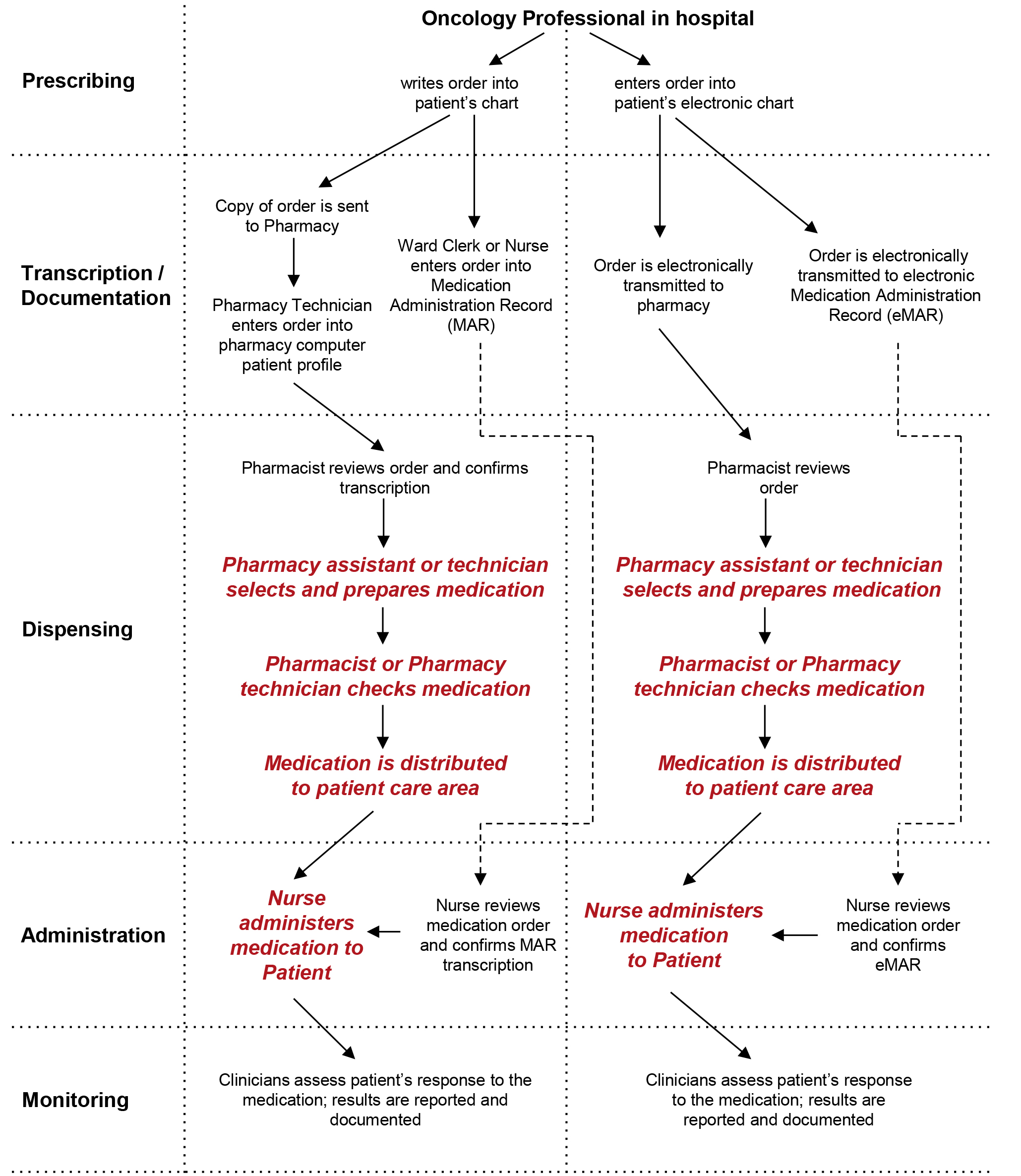
Medication Errors and Adverse Drug Events | PSNet Sep 07, 2019 · Ordering: the clinician must select the appropriate medication and the dose, frequency, and duration. Transcribing: in a paper-based system, an intermediary (a clerk in the hospital setting, or a pharmacist or pharmacy technician in the outpatient setting) must read and interpret the prescription correctly. Medicines: packaging, labelling and patient information leaflets 18 Dec 2014 — Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ... How to Read Medication Orders and Drug Labels - Oregon.gov Labels on prescription medications; ... looks different or is an electronic medical order, it must have the following information: • Name of resident;.13 pages
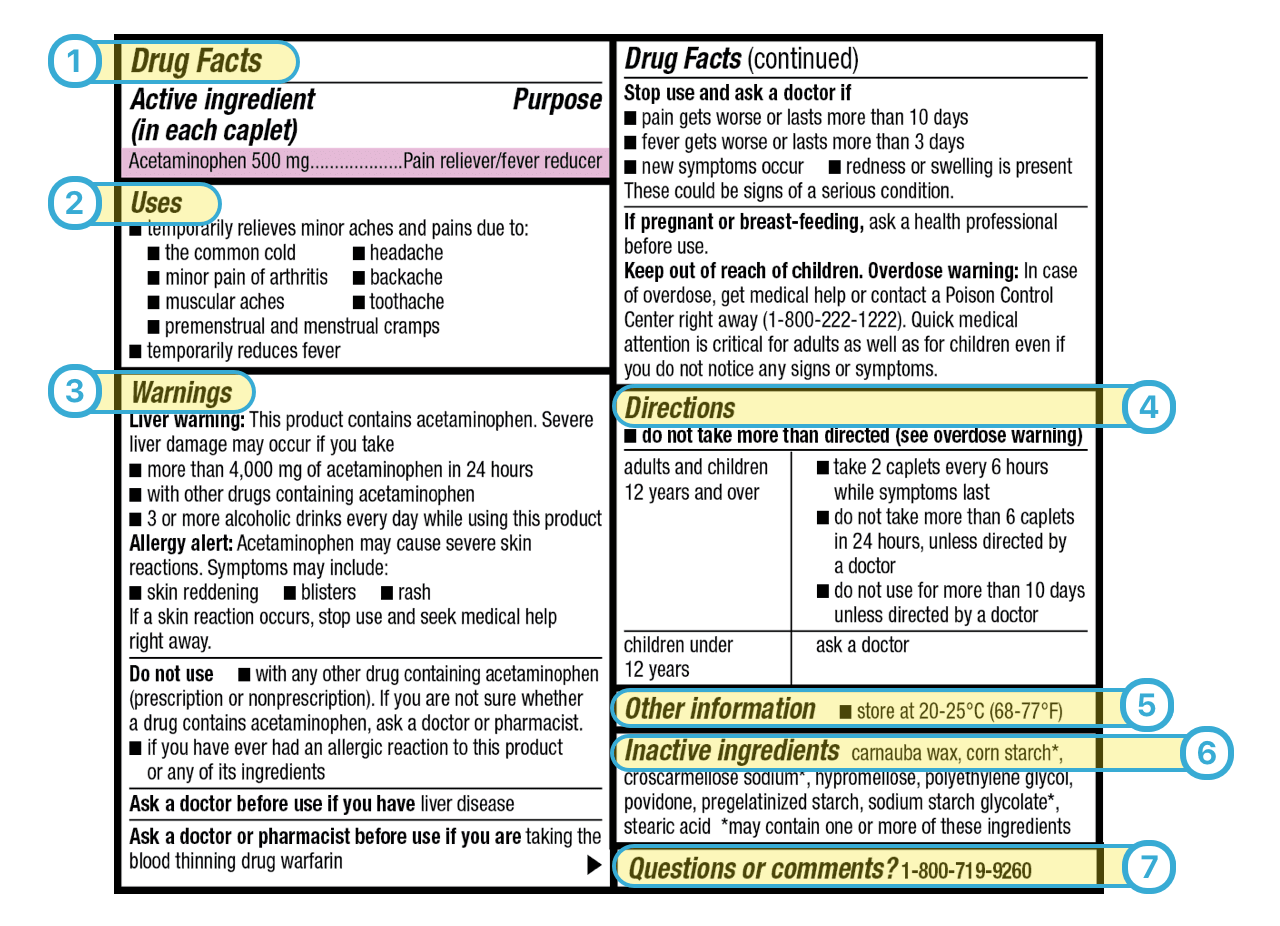
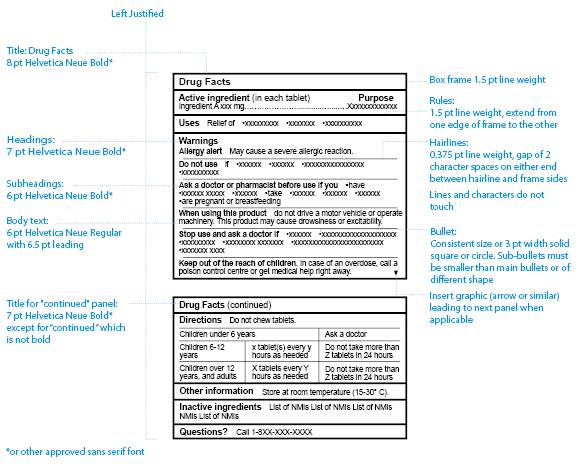
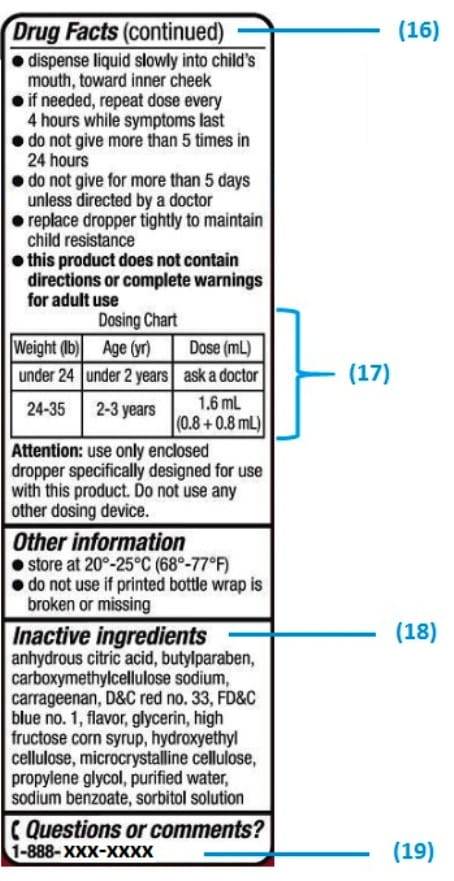
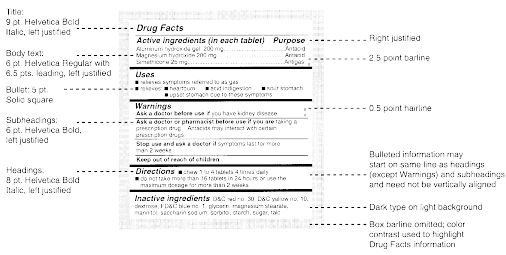
Medication labels must include. Forms in HTML documents - W3 In an HTML document, an element must receive focus from the user in order to become active and perform its tasks. For example, users must activate a link specified by the A element in order to follow the specified link. Similarly, users must give a TEXTAREA focus in order to enter text into it. There are several ways to give focus to an element: Understanding the Critical Requirements for FDA Drug Labels FDA regulations require that all medication labels include: · Name of Product · Table of Drug Facts · Active Ingredients · Proper Use and Purpose · Warnings ... Magnesium - Health Professional Fact Sheet - Magnesium deficiency This is a fact sheet intended for health professionals. For a reader-friendly overview of Magnesium, see our consumer fact sheet on Magnesium.. Introduction. Magnesium, an abundant mineral in the body, is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives). Drug Labeling - StatPearls - NCBI Bookshelf by MJ Lopez · 2021 · Cited by 4 — Definition/Introduction · Highlights (a concise summary of label information) · Full prescribing Information · Limitations Statement · Product Names.
How to Label a Medical Syringe 14 Mar 2022 — Start with the label design and where the information is printed, especially key data like patient name, drug name, dose instructions, dosage ... Forms in HTML documents - W3 In an HTML document, an element must receive focus from the user in order to become active and perform its tasks. For example, users must activate a link specified by the A element in order to follow the specified link. Similarly, users must give a TEXTAREA focus in order to enter text into it. There are several ways to give focus to an element: Best practice guidance on the labelling and packaging of ... Where a generic medicine has a company name included as part of the name registered in section 1 of the SmPC the full colour mock-ups should not include ...16 pages 1910.1030 - Bloodborne pathogens. | Occupational Safety and ... Use.The employer shall ensure that the employee uses appropriate personal protective equipment unless the employer shows that the employee temporarily and briefly declined to use personal protective equipment when, under rare and extraordinary circumstances, it was the employee's professional judgment that in the specific instance its use would have prevented the delivery of health care or ...
Diabetes - Diagnosis and treatment - Mayo Clinic Aug 09, 2022 · They must be counted as part of your meal plan. Understanding what and how much to eat can be a challenge. A registered dietitian can help you create a meal plan that fits your health goals, food preferences and lifestyle. This will likely include carbohydrate counting, especially if you have type 1 diabetes or use insulin as part of your ... Medication Labels 101: Categories, Regulations, and Best ... The basics of medication labels include the drug name, dosage, and directions. Medication labels should always include warnings regarding safety. How to Read Medication Orders and Drug Labels - Oregon.gov Labels on prescription medications; ... looks different or is an electronic medical order, it must have the following information: • Name of resident;.13 pages Medicines: packaging, labelling and patient information leaflets 18 Dec 2014 — Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ...
Medication Errors and Adverse Drug Events | PSNet Sep 07, 2019 · Ordering: the clinician must select the appropriate medication and the dose, frequency, and duration. Transcribing: in a paper-based system, an intermediary (a clerk in the hospital setting, or a pharmacist or pharmacy technician in the outpatient setting) must read and interpret the prescription correctly.











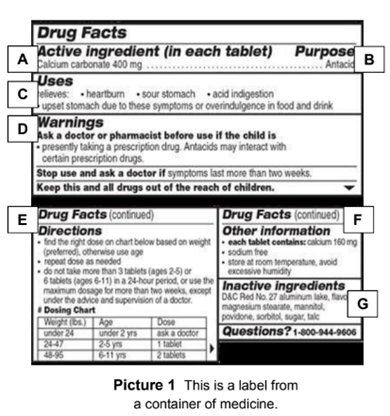


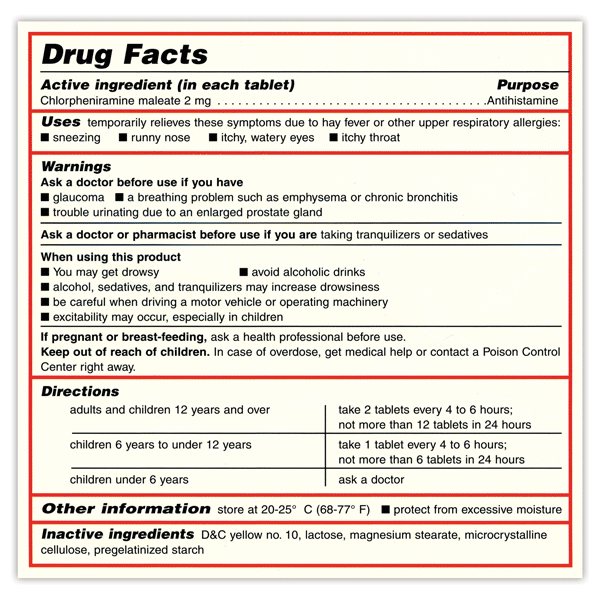






Post a Comment for "41 medication labels must include"