40 fda structured product labels
SPL for FDA Submission - Dakota Systems What is Structured Product Labeling (SPL)? SPL is an HL7 standard that defines the content and structure of product labeling information required for submission to the FDA. It improves the integrity of product information through the use of consistent structure and standard terminology. IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ...
› media › 154586www.fda.gov The FDA-483 document with indicated PRODUCT AREA of DRUGS for: Aetna Felt Corporation 2401 W. Emaus Ave., Allentown, PA, dated: 5/13/2021-5/13/2021 ... structured dataset of when drug applications ...

Fda structured product labels
SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... Structured Product Labeling Validation Rules Structured Product Labeling (SPL) Implementation Guide with Validation Procedures. Technical Specifications Document . This Document is incorporated by reference into the following Guidance ...
Fda structured product labels. Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics... The world of independent media, all in one place. Package Type | FDA NCI Thesaurus OID: 2.16.840.1.113883.3.26.1.1. NCI concept code for package type: C43164 Nsde | Fda CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for...
› current › title-21eCFR :: 21 CFR Part 314 -- Applications for FDA Approval to ... Listed drug status is evidenced by the drug product's identification in the current edition of FDA's “Approved Drug Products With Therapeutic Equivalence Evaluations” (the list) as an approved drug. A drug product is deemed to be a listed drug on the date of approval for the NDA or ANDA for that drug product. FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently... FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents.... Structured Product Labeling Validation Rules Structured Product Labeling (SPL) Implementation Guide with Validation Procedures. Technical Specifications Document . This Document is incorporated by reference into the following Guidance ...
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ...






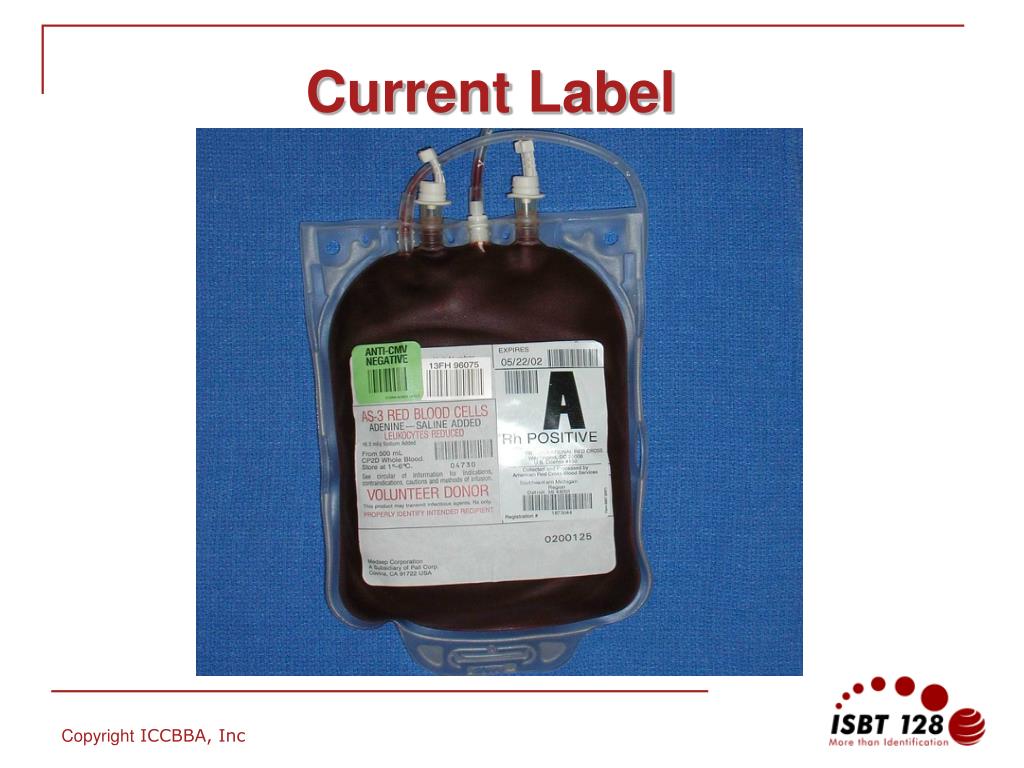


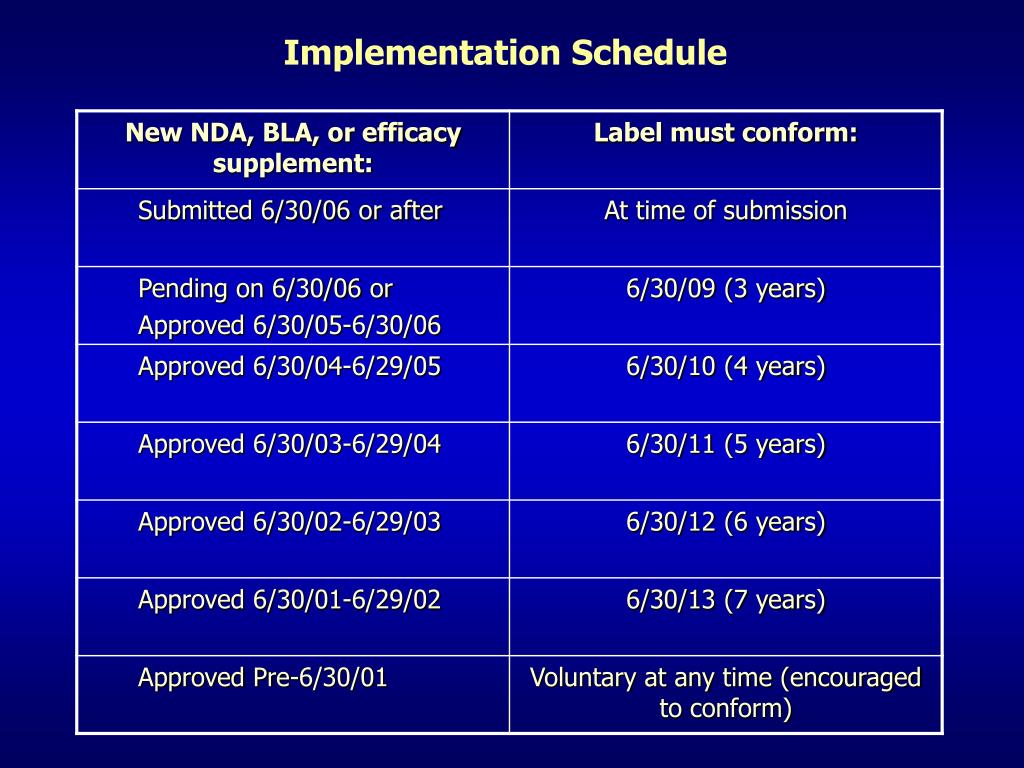
Post a Comment for "40 fda structured product labels"